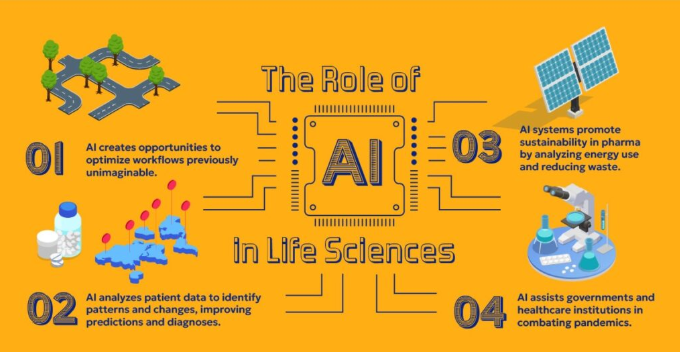
Building a QMS (Quality Management System) for Medical Device
Bringing a medical device to market requires more than innovative design and clinical excellence, it demands a robust, compliant Quality Management System (QMS). ISO 13485:2016 and various regional regulations, such as the FDA’s new Quality Management System Regulation (QMSR), mandate structured documentation, risk management, and continuous quality improvement to ensure…